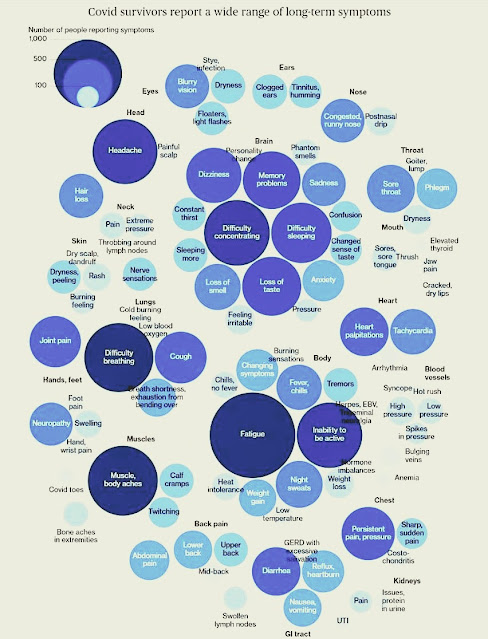
|
| Table 1. Comparison of vaccines |
In the above table, list of Covid-19 vaccines are compared and one of the nonclinical results has mentioned:
- Strong Th1 response (Moderna)
- Strong Th1 and Th2 response (Pfizer, AstraZeneca, J&J and Sputnik V)
Cytokines
- Proinflammatory
- Anti-inflammatory
T Lymphocytes
- Helper T Cells
- T lymphocytes that express CD4
- Killer T Cells
- T lymphocytes that express CD8
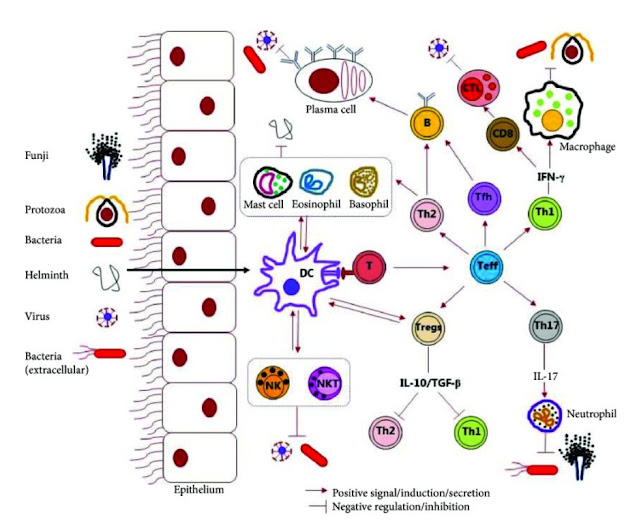 |
| Figure 2. Schematic representation of the host immune response against microbial pathogens (Source: 18). |
Host Immunity
T helper cells (Th cells) are a sub-group of lymphocytes, a type of white blood cell, that play an important role in the immune system, particularly in the adaptive immune system. They help the activity of other immune cells by releasing T cell cytokines. Understanding exactly how helper T cells respond to immune challenges is currently of major interest in immunology.
- Th1
- Th1 is the host immunity against intracellular bacteria and protozoa. Immune stimulation promotes cellular immune system.
- The painful, red swelling around an infected cut or pimple exemplified a Th1 response.
- Th1 cells can be activated by IL-12 and IL-18, or Th17 cells, which respond to other cytokine stimulations such as IL-1β or IL-18 in concert with IL-23 to produce Th-17 associated cytokines.
- COVID-19
- One report has described a higher proportion of IFNγ-producing T helper 1 (Th1)-like cells in patients with moderate disease than in patients with severe disease.[20]
- CD4+ T cells specific for the SARS-CoV-2 spike protein have been identified in acute infection and have a Th1 cell cytokine profile.[21]
- Th2
- Th2 is the host immunity against multicellular helminths (or parasitic worms) and blood-feeding insects. Immune stimulation promotes humoral immune system.
- The itchy red bump of a mosquito bite typified Th2.
- COVID-19
- A role for Th2 cell-type responses in severe COVID-19 is unclear, although patients with mild disease may have a normal TH2 cell response.[22]
Discussions
The human immune system is incredibly complex. We have many types of immune cells that are orchestrated by various factors–from our encounter with microbes, to our health status, genetics, mood, and more.
The main issue with the whole Th1/Th2 theory, as some scientists have recently pointed out, is that the activity of cytokines and other immune messengers rarely fall into strict Th1 or Th2 patterns. Some cells, like non-helper regulatory T cells (Tregs), may influence both Th1 and Th2 responses.[12-14]The optimal scenario would therefore seem to be that humans should produce a well balanced Th1 and Th2 response, suited to the immune challenge.[1]
Many researchers regard allergy as a Th2 weighted imbalance, and recently immunologists have been investigating ways to redirect allergic Th2 responses in favor of Th1 responses to try to reduce the incidence of atopy.
Some groups have been looking at using high dose exposure to allergen to drive up the Th1 response in established disease,[2] and other groups have been studying the use of mycobacterial vaccines in an attempt to drive a stronger Th1 response in early life.[3]
An additional strategy is being used to prevent the onset of disease; this involves the study of pregnancy and early postnatal life. Both of these states are chiefly viewed as Th2 phenomena (to reduce the risk of miscarriage, a strong Th2 response is necessary to modify the Th1 cellular response in utero). The fetus can switch on an immune response early in pregnancy, and because pregnancy is chiefly a Th2 situation, babies tend to be born with Th2 biased immune responses. These can be switched off rapidly postnatally under the influence of microbiological exposure or can be enhanced by early exposure to allergens. It is also hypothesized that those who go on to develop full blown allergies may be those who are born with a generally weaker Th1 response, although it is now apparent that babies with allergies produce weak Th1 and Th2 responses.
Some people have suggested that immunization programmed (and the subsequent reduction in microbiological exposure) are responsible for the increasing incidence of atopy. There is, however, no evidence that immunization causes atopy. Moreover, this is not an argument that we should be exposing children to potentially fatal diseases again. If experiencing native diseases reduces the incidence of atopy, then the task of immunologists must be to develop vaccines that mimic the positive effects of infection.
References
- Th1 and Th2 responses: what are they?
- Gereda JE, Leung DYM, Thatayatikom A, Streib JE, Price MR, Klinnert MD, et al. Relationship between house dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet. 2000;355:1680–1683.
- Jones CA, Holloway JA, Warner JO. Does atopic disease start in foetal life? Allergy. 2000; 55:2–10.
- Can Parasites Heal the Gut?
- Th1 cells switch off Th2 cells and vice versa
- Limitations to the Th1/Th2 model
- CELL-MEDIATED IMMUNITY: Cell-cell interactions in specific immune responses
- Loke Lab - Microbiology
- Can Parasites Heal the Gut?
- Hepatitis A and Allergic Diseases
- Are Dogs More Protective For Children’s Health?
- Th1/Th2 Balance: The Hypothesis, its Limitations, and Implications for Health and Disease
- T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2
- Itch expression by Treg cells controls Th2 inflammatory responses
- All About Regulatory T cells (Tregs) & How to Increase Them
- Th1/Th2 model
- What Are the Different Types of T Cells?
- Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies
- Coronavirus Deranges the Immune System in Complex and Deadly Ways
- Chen, G. et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 130, 2620–2629 (2020).
- Weiskopf, D. et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 5, eabd2071 (2020).
- Laing, A. G. et al. A consensus Covid-19 immune signature combines immuno-protection with discrete sepsis-like traits associated with poor prognosis.
- Pfizer/BioNtech And Moderna MRNA Covid-19 Vaccines Closely Mimic The Immune Response Of Natural SARS-CoV-2 Infections
- COVID vaccines: head-to-head comparison reveals how they stack up







![Understanding Asthma [CHART]](https://www.shape-able.com/wp-content/uploads/Understanding-Asthma-Chart.jpg)