Could Novavax Deliver the Best Coronavirus Vaccine?
(Updated 06/14/2021) The Covid-19 vaccine from American biotech company Novavax reported over 90% efficacy in its phase 3 study—whose results have yet to be peer-reviewed—that prioritized patients typically underrepresented in clinical trials, considered to be at high-risk from coronavirus.
IN EARLY AUGUST, a tiny company won an initial vindication when Novavax announced its strong results from the Australian trial.[3]
Later Australia’s Prime Minister Scott Morrison had announced that:[1]
Australia government would buy 40 million vaccine doses from Novavax and 10 million from Pfizer and BioNTech.
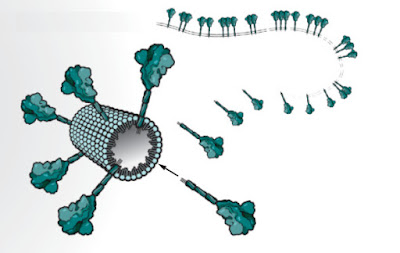
Novavax’s tailormade spike proteins will be mass produced using its unique moth cell system which was developed by Dr. Gale Smith, who had received his Ph.D. from Texas A&M University , with his colleagues.
The company is one of just seven vaccine makers to win funding so far from Operation Warp Speed, the giant multiagency U.S. government effort aiming to quickly produce at least 300 million doses of COVID-19 vaccines. Still, some observers say Novavax’s technology gives it an edge.
Most of Novavax’s key competitors—Moderna, Pfizer, Johnson & Johnson subsidiary Janssen, and AstraZeneca—had launched phase III trials by then. To make their vaccines, all four of those firms use new technologies based on genetic material that directs protein production, rather than delivering proteins directly. Those platforms rely on DNA loaded in disabled viruses or on messenger RNA to carry genetic instructions for building the spike protein. Cells within a vaccinated person then churn out the protein, alerting the immune system.
Protein-based vaccines have a long track record of effectiveness. For example, they include:
Novavax had spent years developing “protein subunit” vaccines, so named because they employ a protein (or part of one) from the targeted virus, plus an adjuvant. For coronavirus, the candidate protein is called spike protein.
Developers of “protein subunit” vaccines must develop their own version of the spike protein—one that closely mimics the naturally occurring spike and is stable enough to retain its immunological punch during manufacturing, packaging, and distribution. Most such vaccines include an additional compound called an adjuvant to help stimulate a strong, protective immune response. Those extra steps make protein vaccines slower to develop than those that deliver genetic instructions.
Figure 1. Individual “nanoparticle” studded with up to 14 tailormade spike proteins.
Amazing Preliminary Clinical Trial Results
The preliminary clinical trial results were really amazing and can be highlighted as the below:
"It’s the only vaccine I’ve seen out of all the candidates that are further down the pipeline that actually had no viral replication in the nasal swabs of vaccinated animals"
"That’s important because stopping viral replication in the nose can reduce the spread of infection among people who may be unaware they are sick."
— Angela Rasmussen, a virologist at Columbia University
- On 03/24/2020[4]
- Novavax' NanoFlu vaccine trial completed by NVAX in March, using the same vaccine production and adjuvant system, gave market beating results in all age groups, including elderly.
- In early August (reports from the phase 1-2 trial)[6]
- After two injections, “the antibody responses in the Novavax paper were markedly stronger than any of the other vaccines that have been reported,” and participants had experienced no severe adverse events.
- Strong results in a dozen monkeys (published on 08/18/2020)[7]
- Monkeys injected with various doses of Novavax’ vaccine and then infected with live coronavirus. The virus failed entirely to multiply in the animals’ noses and replicated in the lungs of just one monkey that received the lowest dose; that animal shut down the infection after 4 days. However, be warned that monkeys are not people.
Novavax
The company is one of just seven vaccine makers to win funding so far from Operation Warp Speed, the giant multiagency U.S. government effort aiming to quickly produce at least 300 million doses of COVID-19 vaccines. Still, some observers say Novavax’s technology gives it an edge.
HHS announced $1.6 billion in funds to support the large-scale manufacturing of Novavax's vaccine candidate. By funding Novavax's manufacturing effort, the federal government will own the 100 million doses expected to result from the demonstration project.
— 07/07/2020Made by moth cells harnessed to crank out the virus’ spike protein—which the pathogen uses to invade human cells—Novavax’ vaccine outshone major competitors on key measures in monkey and early human tests.
Protein-Based vs Gene-Based Vaccines
Protein Subunit Vaccines
Protein-based vaccines have a long track record of effectiveness. For example, they include:
- Hepatitis B vaccine (1986)
- Flu Vaccine (2013)
- Human papillomavirus vaccines (2000s)
in contrast with the newer, largely unproven approaches.
Novavax had spent years developing “protein subunit” vaccines, so named because they employ a protein (or part of one) from the targeted virus, plus an adjuvant. For coronavirus, the candidate protein is called spike protein.
Tailormade Spike Protein
Novavax’s tailormade spike protein, the heart of its vaccine. Tests have shown that the Novavax spike is stable for many weeks at 2°C to 8°C—a key advantage over the Moderna and Pfizer vaccines, which need to be stored at –20°C and –70°C, respectively, and once thawed, last only days in the refrigerator.
Andrew Ward was impressed by its stability and conformation, as well as the vigorous antibody responses it has elicited in humans and animals. “They have the know-how,” he says. “And they obviously, as we confirmed, make a good product.”[8]
References
- India sees early vaccine launch as AstraZeneca deliveries run late (accessed on 11/05/2020)
- Will a small, long-shot U.S. company end up producing the best coronavirus vaccine?
- CLINICAL TRIALS Vaccine Data From Novavax
- Novavax’ NanoFlu Achieves All Primary Endpoints In Phase 3 Clinical Trial
- First-in-Human Trial of a SARS CoV 2 Recombinant Spike Protein Nanoparticle Vaccine
- Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine
- NVX-CoV2373 vaccine protects cynomolgus macaque upper and lower airways against SARS-CoV-2 challenge
- Scientists reveal structural details of spike protein used in leading COVID-19 vaccine
- Deutsche Bank Returns With Updated Primer On Global Race For COVID-19 Vaccine
- Phase 3 trial of Novavax investigational COVID-19 vaccine opens
- How the Novavax Vaccine Works
- Novavax addresses urgent global public health needs with innovative technology